
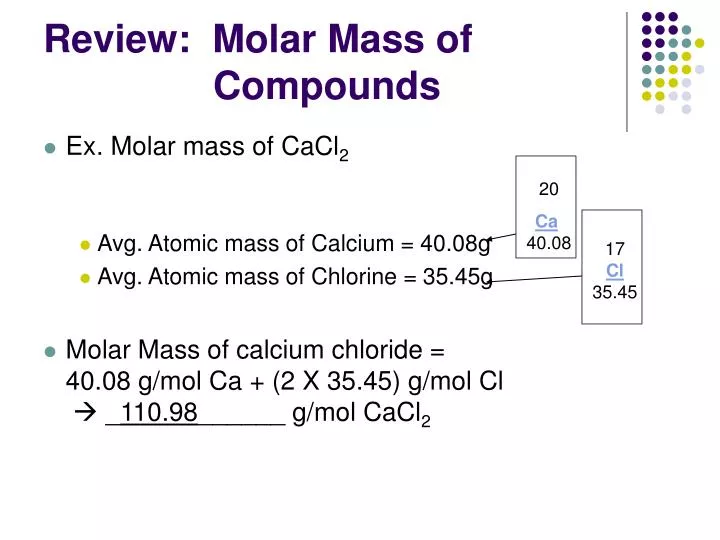
Organic compound having one chlorine atom show molecular peaks Mass spectrum of an organic compound having chlorine atoms also show different For bimolecular ions, ¾ of the chlorine isotopes are Cl 35 and ¼ of Presence of five peaks for chlorine shows the ratio of heights for peaks 1 andĢ is 3: 1. Chlorine has two stable isotopes chlorine-37 (25%) and chlorine -35 (75%).There are five main peaks of isotopes of chlorine of various isotopic monatomic ions. The mass spectrum of chlorine is good example of molecular element. Which help to know relative atomic mass of ionized particle. Sent to computer for analysis and display as mass spectrum. This small current convert into electronic signals appear as ion peaks which Strike the ion detection system where they generate a small electrical current. Ions according to their increasing mass towards the ion detection system. Of ions due to magnetic field is label as R. The moving charge particles create a magnetic fieldĪround itself which interact with magnetic field of the system at point R. Negative plates accelerate the positive ions to Ions to collide with air particles which affect the motion of particles to The low pressure vacuum is needed to stop the The sample must in the gaseous phase, In case The collision of high energy electrons with atoms or molecules causesĪnother electron to remove the particle as positively charged particle. Under analysis and causes ionization of the atoms or molecule form positive High energy electrons from a heated metal element into the vaporized sample High voltage electron gun falls a beam of In the above diagram the symbol K as sampleĪnd Q as high voltage supply label. The substance which is to be analyzed is injected in the high vacuum tube system which has extremely low pressure particles are ionized through colliding with beam of high speed electron. Deflection mass spectrometer consists of ionization, acceleration the positive ions, which in turn deflection of ions and ion detection followed by deflection, separation and detection. TheseĪre different types of mass spectrometer: Method No. Mass spectrometer separates and counts the numbers ofĭifferent positive ions particles are released, the resulting product from theĭetector is known as mass spectrum (plural mass spectra). Vacuum and create electron bombardment to generate a beam of positive ionsĬalled ionization. Mass spectrometry is a technique used to determine relative isotopic masses of different elements and relative abundance of the isotopes.Īll types of mass spectroscopy they include vaporing atoms or molecules in high Mass spectrometry is an important method which is used to identify elements and compounds by their mass spectrum. But Cl + ions will pass through the machine and give lines at 35 and 37 it is depend upon isotopes. The Cl atom is neither accelerated nor deflected in the machine it is not ionized in the ionization chamber and simply lost. The ions are not stable so some will formĬhlorine atom and a Cl + ion. Into the ionization chamber, the electrons are knocked off, and give molecular
It consist of molecules so when it passed It has two isotopes Cl-35 and Cl-37, so it containģ atoms of Cl-35 and 1 atom of Cl-37. The mass spectrum of ChlorineĬhlorine is such an element which contain more

– 37, the weighted average is closer to 35 than 37. Chlorine -35 is about 3 times more abundant than chlorine

Has a relative abundance of 75.76% ,whereas chlorine – 37 has a relativeĪbundance of 24.24%. Different isotopes have different relative abundances ,chlorine – 35 The atomic weight of chlorine given on the periodic table isģ5.47 u. The seven electrons in the third outermost shell acting as its valenceĬhlorine has two stable isotopes: chlorine – 35Īnd chorine – 37. Chlorine hasĮlectronic configuration 3s 23p 5 with Has similar properties like fluorine, bromine and iodine. Chlorine is the second member of halogen group it If the litmus paper is bleached white then the gas is Chlorine or Fluorine.And it has atomic number 17.Place a piece of litmus paper over the mouth of the test tube.Chlorine is a pale green coloured gas at standard temperature and pressure. Chlorine reacts strongly with Hydrogen to produce Hydrogen Chloride which dissolves in water to produce Hydrochloric Acid. Chlorine is a more reactive Halogen than Bromine but less reactive than Fluorine. Properties Chlorine is a non-metal element. A diagram showing the formation of a Chloride ion.


 0 kommentar(er)
0 kommentar(er)
